|
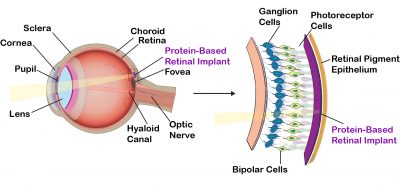
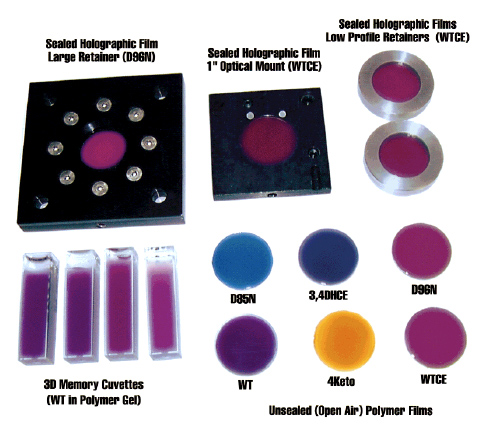
Although nature has optimized the native protein bacteriorhodopsin for photochemical stability and proton pumping efficiency, evolution does not optimize proteins for device environments. Directed evolution has long been known as a powerful method of optimizing enzymes, but relatively little success has been found in using directed evolution to optimize photochromic proteins. The problem is due in large part to the difficulty of selecting mutants that demonstrate the desired property or behavior. Dr. Nicole Wagner in our group has developed high-throughput, automated methods of selection that permit the efficient and iterative selection of successful mutations. We have optimized the photophysical properties of bacteriorhodopsin for use in a number of device applications, including three-dimensional optical memories (Wise et al., 2002 ), Fourier transform holographic associative processors (Hillebrecht et al., 2005 ), Fourier transform holographic associative processors (Hillebrecht et al., 2005 ; Ranaghan et al., 2014 ; Ranaghan et al., 2014 ), photovoltaic systems (Wise et al., 2002 ), photovoltaic systems (Wise et al., 2002 ) and retinal implants (Wagner et al., 2013 ) and retinal implants (Wagner et al., 2013 ). ).
|